Research Goal
Our goal is to provide a comprehensive understanding of how the brain works and the causes of brain dysfunction. We use mouse models to decipher how malfunction of neuronal circuits underlie neuropsychiatric disorders. Our approaches bridge the scales of gene expression mechanisms, synaptic function, neuronal ensemble activity and behavior. The impact of our research is an hypothesis-driven experimentally-tested insight to inform corrections of brain circuit function and treatments to improve cognitive outcome.
Current Projects
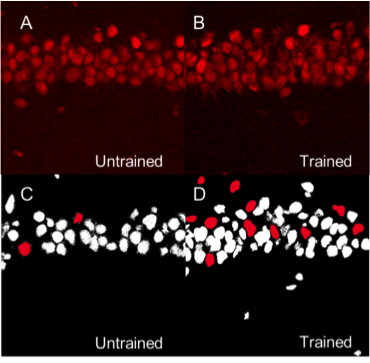
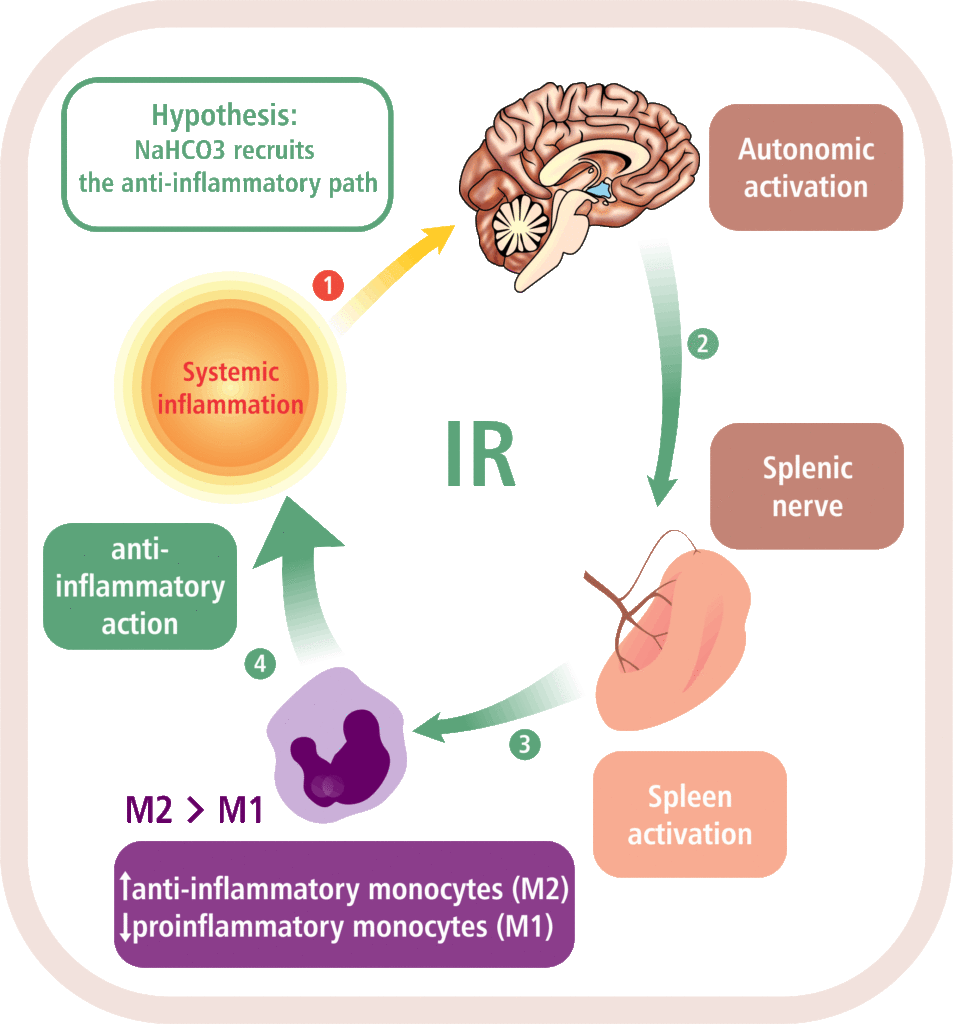
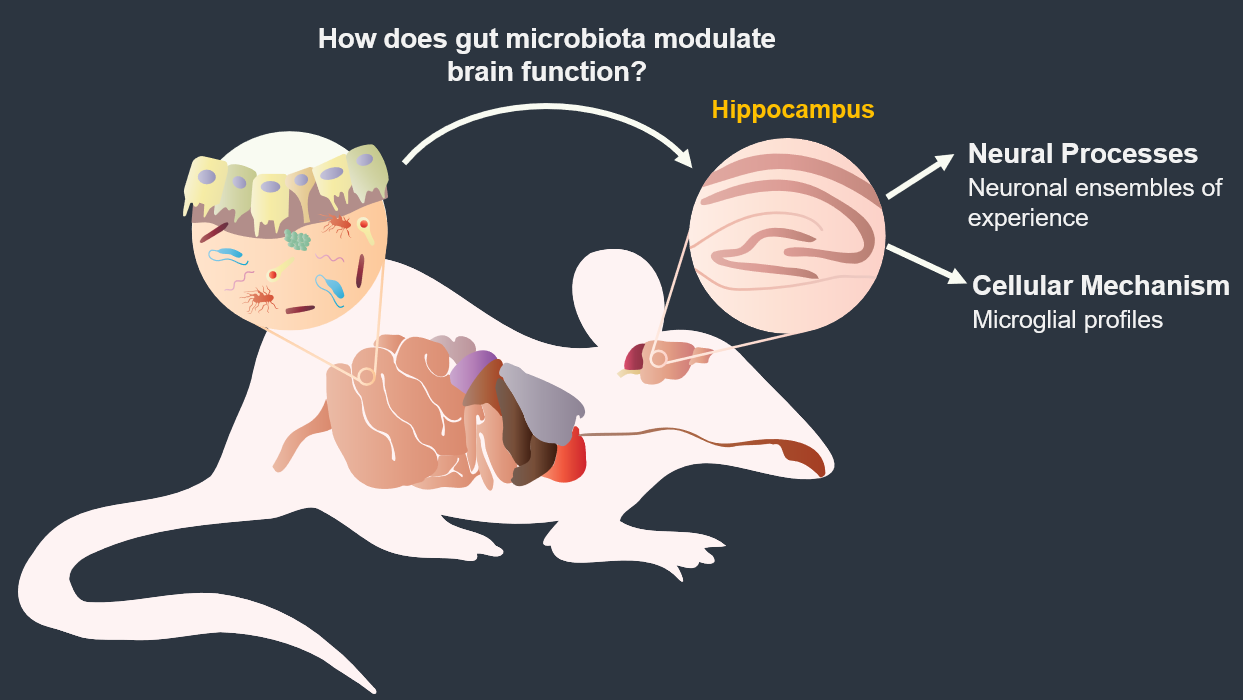
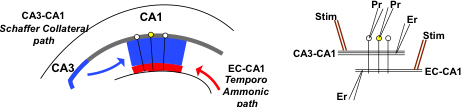
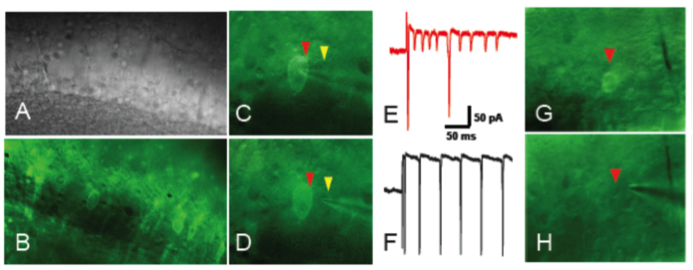
Mapping memory traces within the hippocampal network How the brain changes with memory is a fundamental question in neuroscience. This question entails deciphering how experience sculpts neural circuits to store memory. To meet this challenge, our research program uses a combination of behavioral, molecular, genetic and electrophysiological approaches to map the neuronal, functional and mechanistic organization of memory traces in the hippocampal network. In vivo immediate early gene tagging, synaptic function characterization and spatial transcriptomics RNA sequencing methodologies will allow us to test the hypothesis that memory experiences shape hippocampal circuits with gene expression, circuit function and neuronal ensemble architecture that is unique to each experience. The overall impact of this research is to build a new framework that integrates the scales of mechanisms, function and neuronal ensembles for a comprehensive understanding of the biology of memory. Modulation of the inflammatory reflex:impact on the gut-brain-spleen axis Uncontrolled systemic inflammation is the hallmark of many acute and inflammatory conditions including COVID19, sepsis, stroke, heart disease, type 2 diabetes, cancer, autoimmune diseases, among others. Available therapies are expensive and expose patients to lethal infections. An alternative approach is the stimulation of the inflammatory reflex (IR), which engages the brain-spleen axis to homeostatically downregulate systemic inflammation. Recent findings suggest that oral intake of sodium bicarbonate (NaHCO3) triggers IR activation. This proposal seeks to characterize the neural mechanism mediating NaHCO3’s action on the IR (box). The hypothesis that NaHCO3-induced IR activation requires intact spleen innervation and engages specific autonomic brain areas, will be tested by spleen denervation, flow cytometry analysis, and immunofluorescence techniques. Successful accomplishment of this proposal could establish NaHCO3 as a safe and cost-effective method to activate the IR, laying the groundwork for translational and clinical studies to treat systemic inflammatory conditions. Gut microbiota modulation of neuronal and microglial function Trillions of microbes —comprising communities of bacteria, virus, fungi, and other microorganisms— inhabit the gastrointestinal tract. Known as “gut microbiome”, these microorganisms are central to human health. Diet, exercise, prescription drugs and environment, among other factors, largely influence the composition of the gut microbiome and imbalances of these microorganisms’ communities are associated with several diseases, including neuropsychiatric conditions. A number of studies in laboratory animals connect gut microbes with processes and mechanisms of learning and memory. Experience activates subsets of neurons in the brain leaving a footprint. Known as memory trace, these ensembles are modulated by microglial activity. Gut microbiome is paramount for proper function and maturation of microglia and microglia dysfunction is found in brain disease. Whether gut microbes modulate the allocation of neurons into the memory trace and impacts microglia-neuron interaction within the memory trace has been largely unexplored. The proposal investigatesthe impact of microbiome content on the spatial organization of neuronal ensembles and microglia in the rodent hippocampus. Impacts of early life stress on the neurobiology of depression The goal of this project is to examine the impact of early life stress (ELS) on the brain areas and pathways implicated in depression (also used for threat appraisal). Half of depressed patients do not respond to treatment, and in this population, most show different manifestations of early life stress. ELS could cause longstanding changes in neurons and pathways that are currently unknown. Understanding these impacts could lead to novel treatments both behavioral and pharmacological. Experience-dependent modulation of synaptic circuits in the hippocampus It is generally accepted that when we learn, our brain changes in order to store a memory. Much is known on the identification of brain regions and mechanisms important for the storage of memory. For instance, memories of facts and episodes (e.g. spatial memory) largely depend on the activity of the hippocampus. Similarly, synaptic plasticity, the experience-dependent change in synaptic function, is thought to be the cellular mechanism that underlies memory formation. Yet, we still lack a detailed understanding of the organization of memory traces at the level of neural and synaptic circuits. Our goal is to define how storing memories changes the function of neurons and their synaptic circuits in the hippocampus of mice that have been trained in a spatial task. We move toward this goal by way of an exciting interdisciplinary research collaboration with the laboratory of Dr. André A. Fenton (NYU). We investigate how learned experiences persistently modify the function of neurons in the CA1 area of the hippocampus by testing the electrophysiological properties of their intrahippocampal (CA3-CA1) and cortical (EC-CA1) synaptic circuits (Fig. 1). To identify neurons potentially recruited into the memory trace, we use a transgenic mouse that allows us to temporally restrict the expression of an immediate early gene-driven fluorescent protein during spatial learning (Fig. 2A-B). In addition to the electrophysiological analysis (Fig. 2C-F), we determine changes in mRNA expression and protein levels in single neurons (Fig. 2G-H). This combined analysis allows us to explore a connection between functional and molecular changes of neurons that putatively form a memory trace. Neural ensembles are speculated to support memory traces at the systems level. This project aims to bridge the scales of neural ensembles (connectome) and synaptic circuits (synaptome) by defining the changes of synaptic circuit function and mechanisms that underlie ensemble activity thought to generate a memory trace. Figure 1. Left: CA3-CA1 synaptic inputs at the stratum radiatum (blue), EC(layer III)-CA1 synaptic inputs at the stratum lacunosum moleculare (red). Right: Stimulation electrodes (stim; brown) activate each input. Extracellular electrodes (Er) record synaptic potentials from each path. Patch electrodes (Pr) record from CA1 principal neurons. Figure 2. Examination of learning-associated neurons in Arc/Cre/YFP mice, 1 day after spatial training. A: Bright field image of the CA1 pyramidal layer. B: Fluorescent imaging reveals a subset of neurons with YFP positive labeling (bright green). Arc-driven YFP expression is permissive only in neurons that underwent strong neural activity (possibly associated with learning). C-F: The approach allow us to identify and patch-clamp YFP-labeled (red arrow) and unlabeled (yellow arrow) neurons. G,H: Identified neurons can then be micro-aspired for single-cell PCR analyses. Credits: Work by Ain Chung (NYU, Fenton Lab) and Alarcon Lab. Decoding place cell firing-induced synaptic plasticity and cognitive mapping The goal of this project is to characterize the core features of spike code that are important for the formation of synaptic plasticity. Synaptic plasticity is an experience-dependent change in the function of synapses and is hypothesized to be an important mechanism for learning and memory processes. When an animal moves through an environment, particular neurons in the hippocampus fire action potentials only at specific locations of the environment; these neurons are known as “place cells.” Figure 1. A: Place cell firing fields of cell 1A and cell 1B. B: Time stamps representing spike times for each place cell (1A and 1B). C: In vitro setup. Time stamps for place cell spikes are used to form sequence of 0 and 1 values in computer memory that are transformed into pulses by digital-to-analog (D/A) converters; the D/A outputs, in turn, drive stimulators. In C, Schaffer collateral stimulation is controlled by the activity of the blue cell; current passed through the patch electrode elicits post-synaptic spikes according to the activity of the red cell. D: (Top left) Excitatory postsynaptic current (EPSC) occurrence and (top right) EPSC failure observed with the minimal stimulation method described in Isaac et al. (2009). (Bottom) Single synaptic site stimulation is confirmed by absence of paired-pulse facilitation of the EPSC at 20 milliseconds inter-pulse interval. [A-C from Isaac et al., 2009.] Compartmentalized synaptic plasticity Neurons in the brain receive multiple streams of information from other neurons via synaptic connections. How do neurons integrate the multitude of incoming information arriving at their synapses is key to understand how processing of information occurs in the brain. Figure 1. Left: Schematic of slice preparation and major afferent inputs. DG, dentate gyrus; SO, stratum oriens; SR, stratum radiatum; SLM, stratum lacunosum moleculare. Right: Photomicrograph of CA1 area. SR, stratum radiatum; SP, stratum pyramidale; re, recording electrode; se, stimulating electrode. Molecular mechanisms underlying learning and memory A long standing research collaboration with Dr. Ivan Hernandez’s laboratory, this project aims to investigate the mechanisms that underlie learning and memory processes. Physiological and molecular evidence indicate that synaptic plasticity, a cellular phenomenon known to modulate synaptic function, may be the cellular substrate of learning and memory processes. Indeed, the Synaptic Plasticity Hypothesis of Memory states that mechanisms supporting long-lasting synaptic plasticity are fundamental to the maintenance of memory. Figure 1. Changes in CA1 PARP activity after memory training. A,B: Immunohistochemical images for PARP in the hippocampus CA1 area from an untrained and a trained mouse. C,D: Imaging spectral analysis of A and B. Cells nuclei with higher PARP activity are colored in red. The analysis shows that 20-30% of imaged neurons have high PARP activity, suggesting that these cells may have been potentially engaged in memory-associated processes. Note that only a small population (~5%) of nuclei from an untrained mouse shows high PARP activity. Credits: Immunohistochemistry and imaging analysis carried out by Christienne Damatac and Amanpreet Kandola at Alarcon’s Lab.
Past Projects
A brainchild of the late Professor Robert L. Muller (SUNY Downstate), this study investigates how sequences of spike activity (action potentials), recorded from place cells, change the activity of synapses in a brain slice preparation. Our approach, developed by Isaac et al. (2009) and Froemke and Dan (2002), utilizes the time series of place cell spiking originally recorded from the freely moving animal as input stimulation to the pre and postsynaptic elements of CA1 pyramidal cells (Fig. 1). That is, we reproduce in a brain slice preparation the synaptic behavior of two synaptically connected place cells as we speculated it occurs in the behaving animal.
Our work is expected to aid in understanding aspects of spike coding information, such as frequency of spiking (rate spike coding) or timing of spiking (temporal spike coding), that are important for the formation of synaptic plasticity needed for the encoding of navigational information.![Figure 1. A: Place cell firing fields of cell 1A and cell 1B. B: Time stamps representing spike times for each place cell (1A and 1B). C: In vitro setup. Time stamps for place cell spikes are used to form sequence of 0 and 1 values in computer memory that are transformed into pulses by digital-to-analog (D/A) converters; the D/A outputs, in turn, drive stimulators. In C, Schaffer collateral stimulation is controlled by the activity of the blue cell and current passed through the patch electrode elicits post-synaptic spikes according to the activity of the red cell. D: (Top left) Excitatory postsynaptic current (EPSC) occurrence and (top right) EPSC failure observed with the minimal stimulation method described in Isaac et al. (2009). (Bottom) Single synaptic site stimulation is confirmed by absence of paired-pulse facilitation of the EPSC at 20 milliseconds inter-pulse interval. [A-C from Isaac et al., 2009.]](http://www.alarconlab.com/wp-content/uploads/2014/05/P2F1.png)
Synaptic plasticity is an experience-dependent modulation of the function of synapses and is thought to be an important mechanism for the process of learning and the formation of memory. During learning, it is speculated that multiple streams of learning-associated neural activity arrive and trigger synaptic plasticity mechanisms at distinct synapses of the neuron.
Our goal is to define how a neuron computes information when multiple forms of synaptic plasticity mechanisms are activated in two or more of its synapses. Our work is inspired by the synaptic tagging and capture hypothesis developed by Frey and Morris (1997, 1998). We investigate the computing capability of CA1 neurons of the mouse hippocampus that have been stimulated at separate synaptic inputs to induce different forms of synaptic plasticity (Fig. 1).
Our studies suggest that activation of synaptic plasticity mechanisms at a given synaptic input can change the properties of neighboring (unstimulated) synapses; a phenomenon that we call “compartmentalized synaptic plasticity” (Pavlowsky and Alarcon, 2012).
Our work investigates whether compartmentalization is an important process for the computing capability of neurons that is required to organize distinct learning-associated streams of information arriving to a neuron. We think these compartments work as units of computation for the association/separation and inclusion/dismissal of learning and memory information.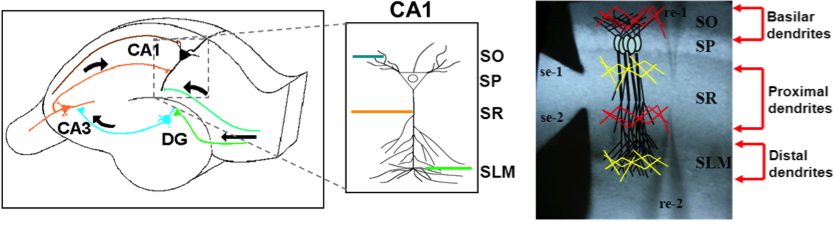
Molecular neuroscience studies at Hernandez’s lab center on identifying a “master switch” mechanism able to control gene expression and protein synthesis relevant for the maintenance of synaptic plasticity and memory. Together, we observed that the requirement for poly(ADP)ribose polymerase (PARP) activity, an enzyme that regulates gene expression via chromatin relaxation, can distinguish populations of CA1 neurons of the hippocampus of mice trained in a spatial memory task versus those of untrained mice (Fig. 1). The identification of learning-associated molecular changes has initiated a line of research that is expected to bridge cell-specific molecular mechanisms and memory traces in cell networks.